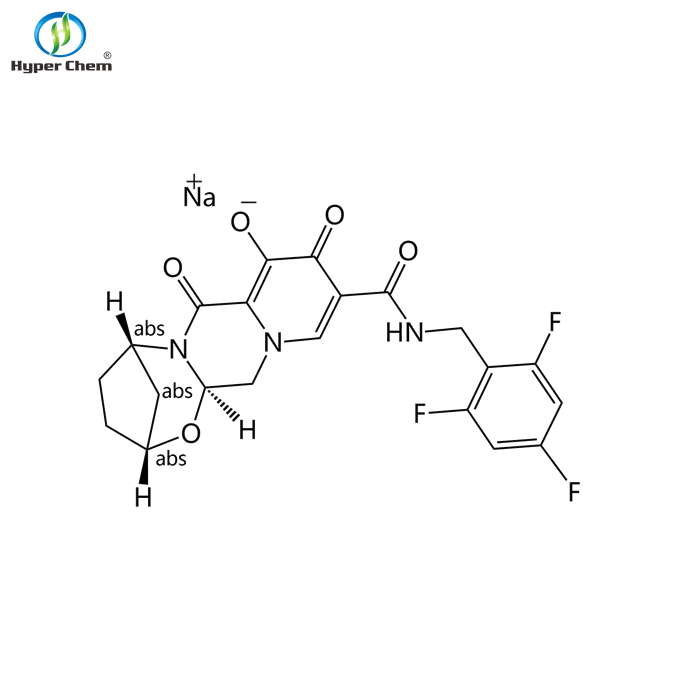
CAS 1807988-02-8 Bictegravir Sodium
Quick Details Port: Any port in China Payment Terms: L/C,D/P,T/T,Western Union,MoneyGram Supply Ability: 1 Kilogram/Kilograms per Month Bictegravir Sodium, CAS 1807988-02-8, Medicine Grade Quality standard: Purity 99% min Other Names: 1807988-02-8 Grade Standard: Medicine Grade Purity: 99% min Grade: Pharma Grade Appearance: White powder,White powder CAS No.: 1807988-02-8 Type: API EINECS No.: N/A MF: C21H17F3N3NaO5 Application: HIV-1 and HIV-2 infection Agents Packaging Detail: For small quantity: Bag Commercial quantity: None Or as per customers required Identification Name R&D Use only,Bictegravir Sodium, CAS 1807988-02-8 Structure Other name 1807988-02-8Bictegravir sodium Key words API, treatment of HIV-1 and HIV-2 infection. It has been approved for HIV-1 monotherapy combined with 2 other antiretrovirals in a single tablet.Bictegravir Sodium, CAS 1807988-02-8Bictegravir Sodium, CAS 1807988-02-8 supplier, Bictegravir Sodium, CAS 1807988-02-8 distributor, CAS 328898-40-4Bictegravir Sodium, CAS 1807988-02-8 manufacturer, Bictegravir Sodium, CAS 1807988-02-8 wholesale;China Bictegravir Sodium, CAS 1807988-02-8 manufacturers;Bictegravir Sodium, CAS 1807988-02-8 cost,Bictegravir Sodium, CAS 1807988-02-8 buy,Bictegravir Sodium, CAS 1807988-02-8 sale CAS NO. 1807988-02-8 Molecular Formula C21H17F3N3NaO5 Molecular Weight 471.368 g/mol Chemical and Physical Properties Appearance White powder Quality standard Purity 99% min Application Drug Indication Bictegravir is indicated in the management of HIV-1 infection in patients not previously treated with antiretroviral therapy. Formulations/Preparations For […]